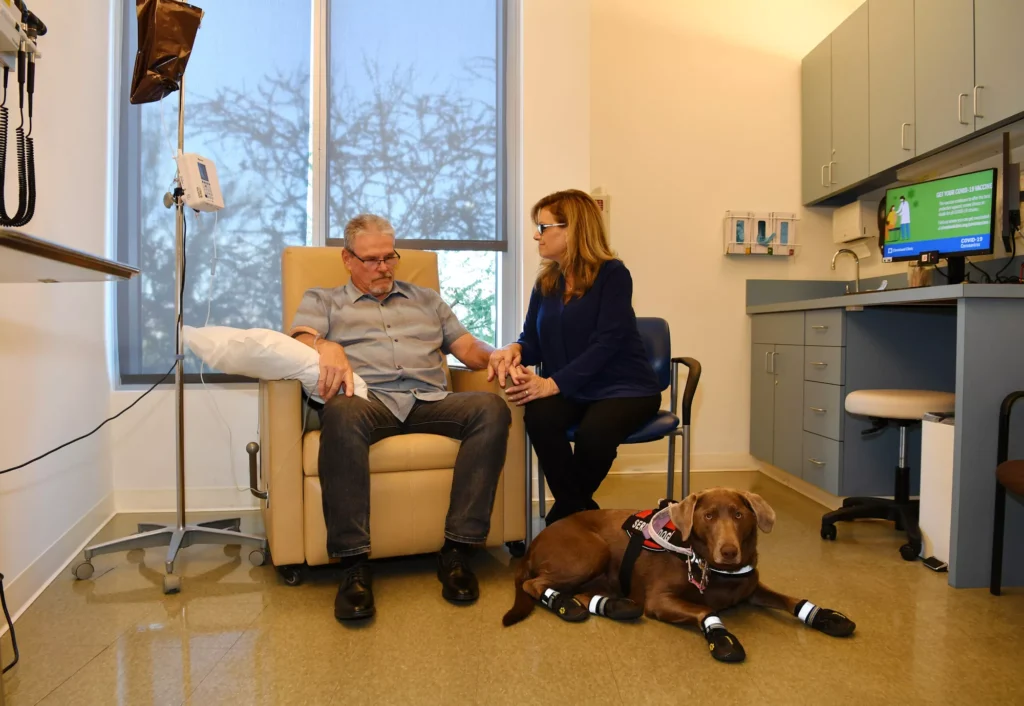
On November 2, 2023, Cleveland Clinic Lou Ruvo Center for Brain Health achieved a milestone in Alzheimer’s care by administering its first infusion of lecanemab (LEQEMBI®), an FDA-approved anti-amyloid drug for mild Alzheimer’s disease. This event coincided with National Alzheimer’s Disease Awareness Month and marked the first time the center provided a treatment proven to slow disease progression rather than only managing symptoms.
The Lou Ruvo Center, a leading Alzheimer’s clinical trial site, has participated in the phase 3 CLARITY study of lecanemab since 2020 and continues to study the drug in the AHEAD trial for individuals at risk of dementia. It remains the sole Nevada site for these trials.
Dan Harrington, a 64-year-old Alzheimer’s patient who relocated to Las Vegas for care at the center, expressed gratitude for the opportunity to try the groundbreaking therapy. “There’s so much hope,” said his wife, Andrea Harrington. “We can continue to make memories and enjoy what we have.”
“This treatment is a significant step forward,” said Dr. Dylan Wint, director of the Lou Ruvo Center for Brain Health. “While it won’t solve the Alzheimer’s crisis, it slows progression and delays some devastating symptoms.”
The infusion represents a new chapter in Alzheimer’s care, offering hope to patients and families. Andrea Harrington added, “If we can inspire others to seek treatment and not be afraid, that’s what we’ll do.”
